Worksheet naming molecular compounds answers provides a comprehensive guide to the fundamental principles and applications of naming molecular compounds. This resource offers a structured approach to understanding the IUPAC nomenclature system, empowering students with the knowledge and skills to accurately name and identify molecular compounds.
Delving into the intricacies of chemical nomenclature, this worksheet covers the rules and conventions for naming binary molecular compounds, molecular compounds containing polyatomic ions, acids, bases, and salts. Through clear explanations and illustrative examples, learners will gain a thorough understanding of the systematic approach to naming molecular compounds.
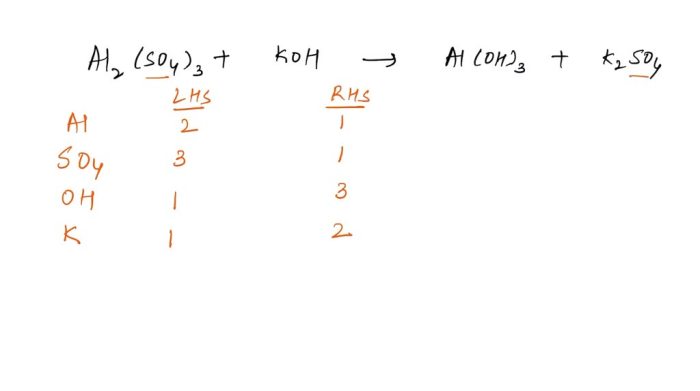
Nomenclature of Molecular Compounds

Molecular compounds are composed of two or more nonmetal atoms. The rules for naming molecular compounds are as follows:
- The first element in the name is the one that appears first in the formula.
- The second element in the name is the one that appears second in the formula.
- The suffix “-ide” is added to the name of the second element.
- Prefixes are used to indicate the number of atoms of each element in the compound. The prefixes are:
- mono-
- di-
- tri-
- tetra-
- penta-
- hexa-
- hepta-
- octa-
- nona-
- deca-
For example, the compound with the formula CO 2is called carbon dioxide. The first element in the formula is carbon, so the first element in the name is carbon. The second element in the formula is oxygen, so the second element in the name is oxygen.
The suffix “-ide” is added to the name of the second element, so the name of the compound is carbon dioxide.
Naming Molecular Compounds with Polyatomic Ions
Polyatomic ions are ions that contain more than one atom. The rules for naming molecular compounds with polyatomic ions are as follows:
- The first element in the name is the one that appears first in the formula.
- The second element in the name is the one that appears second in the formula.
- The suffix “-ate” or “-ite” is added to the name of the second element, depending on the oxidation state of the central atom.
- Parentheses are used to enclose polyatomic ions in the name of a compound.
For example, the compound with the formula NaCl is called sodium chloride. The first element in the formula is sodium, so the first element in the name is sodium. The second element in the formula is chlorine, so the second element in the name is chlorine.
The suffix “-ide” is added to the name of the second element, so the name of the compound is sodium chloride.
Naming Acids
Acids are compounds that donate protons (H+ ions). The rules for naming acids are as follows:
- The name of an acid starts with the word “hydro-“.
- The second part of the name is the name of the anion, with the suffix “-ic” or “-ous” added to indicate the oxidation state of the central atom.
For example, the acid with the formula HCl is called hydrochloric acid. The first part of the name is “hydro-“, which indicates that the acid is a proton donor. The second part of the name is “chloric”, which indicates that the central atom in the anion is chlorine and that it is in the +7 oxidation state.
Naming Bases
Bases are compounds that accept protons (H+ ions). The rules for naming bases are as follows:
- The name of a base starts with the word “hydroxide”.
- The second part of the name is the name of the metal, with the suffix “-ate” or “-ite” added to indicate the oxidation state of the metal.
For example, the base with the formula NaOH is called sodium hydroxide. The first part of the name is “hydroxide”, which indicates that the base is a proton acceptor. The second part of the name is “sodium”, which indicates that the metal in the base is sodium.
Naming Salts, Worksheet naming molecular compounds answers
Salts are compounds that are formed when an acid and a base react. The rules for naming salts are as follows:
- The name of a salt starts with the name of the cation.
- The second part of the name is the name of the anion.
For example, the salt with the formula NaCl is called sodium chloride. The first part of the name is “sodium”, which is the name of the cation. The second part of the name is “chloride”, which is the name of the anion.
Top FAQs: Worksheet Naming Molecular Compounds Answers
What is the purpose of worksheet naming molecular compounds answers?
Worksheet naming molecular compounds answers provides a structured and comprehensive guide to the IUPAC nomenclature system, enabling students to accurately name and identify molecular compounds.
What topics are covered in this worksheet?
This worksheet covers the rules and conventions for naming binary molecular compounds, molecular compounds containing polyatomic ions, acids, bases, and salts.
How can this worksheet benefit students?
By completing this worksheet, students will gain a thorough understanding of the systematic approach to naming molecular compounds, enhancing their communication skills and academic performance in chemistry.