Embark on a comprehensive exploration of matter through the lens of chemistry with our engaging Classification of Matter Worksheet Chemistry. Dive into the fundamental concepts that govern the behavior and properties of matter, unraveling the intricacies of its states, classification, changes, and separation.
This worksheet provides an in-depth examination of the three main states of matter—solid, liquid, and gas—along with their defining characteristics and the intermolecular forces that shape them. Delve into the classification of matter, distinguishing between pure substances and mixtures, elements, compounds, and mixtures, with illustrative examples to solidify your understanding.
States of Matter: Classification Of Matter Worksheet Chemistry
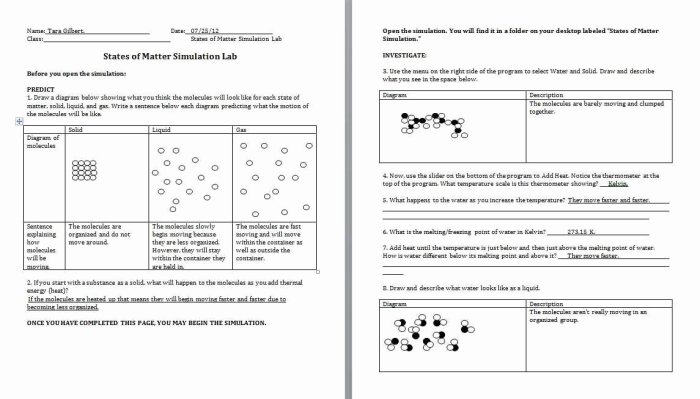
Matter exists in three primary states: solid, liquid, and gas. Each state exhibits distinct characteristics due to the arrangement and interaction of its molecules.
Solidshave a fixed shape and volume. Their molecules are tightly packed, forming a rigid structure. Examples include ice, metal, and wood.
Liquidshave a fixed volume but no definite shape. Their molecules are closely spaced but can move past each other, allowing liquids to flow. Examples include water, oil, and milk.
Gaseshave neither a fixed shape nor volume. Their molecules are widely spaced and move freely, allowing gases to expand to fill their container. Examples include air, helium, and hydrogen.
The state of matter is influenced by intermolecular forces, which are the attractive or repulsive forces between molecules. Stronger intermolecular forces lead to a more condensed state (solid), while weaker forces result in a more dispersed state (gas).
Classification of Matter, Classification of matter worksheet chemistry
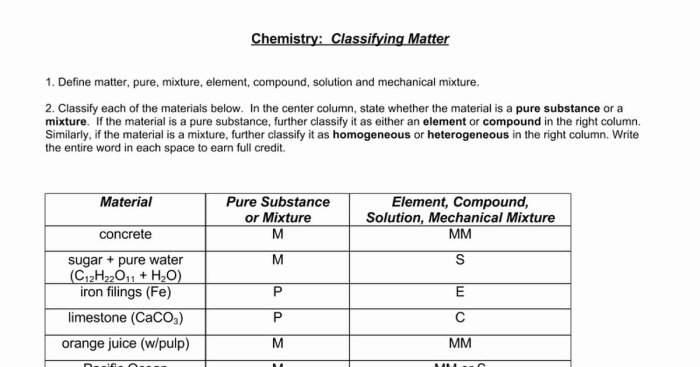
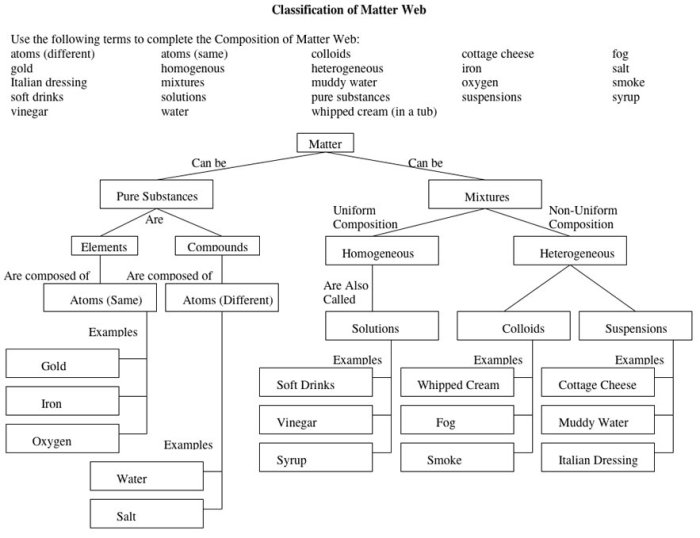
Matter can be classified as either a pure substance or a mixture.
Pure substancesare composed of only one type of atom or molecule. They can be either elements or compounds.
Elementsare the simplest form of matter and cannot be broken down into simpler substances by chemical means. Examples include gold, oxygen, and hydrogen.
Compoundsare composed of two or more elements chemically combined in a fixed ratio. Examples include water (H 2O), salt (NaCl), and sugar (C 12H 22O 11).
Mixturesare combinations of two or more pure substances that are not chemically combined. They can be either homogeneous or heterogeneous.
Homogeneous mixtureshave a uniform composition throughout. Examples include saltwater and air.
Heterogeneous mixtureshave a non-uniform composition, with different regions having different properties. Examples include sand in water and oil in water.
Physical and Chemical Changes
Matter can undergo two types of changes: physical changes and chemical changes.
Physical changesalter the physical properties of a substance without changing its chemical composition. Examples include melting, freezing, and boiling.
Chemical changesinvolve the rearrangement of atoms to form new substances. Examples include burning, rusting, and digestion.
Physical changes are typically reversible, while chemical changes are irreversible.
Separation of Mixtures
Mixtures can be separated into their component parts using various methods, depending on the properties of the substances involved.
Filtrationseparates solids from liquids using a filter paper or membrane. The solid particles are trapped on the filter, while the liquid passes through.
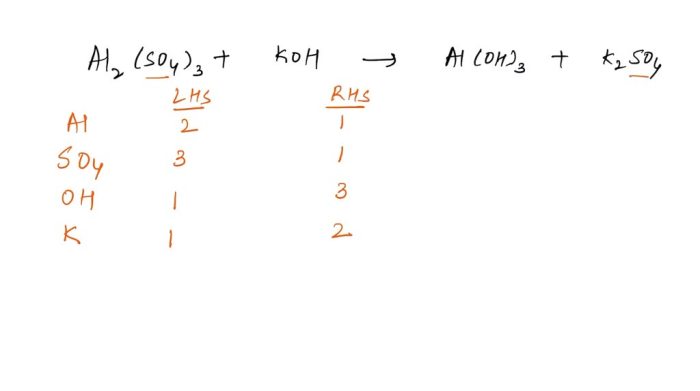
Distillationseparates liquids based on their boiling points. The mixture is heated, and the liquid with the lower boiling point evaporates first. The vapor is then condensed and collected.
Chromatographyseparates substances based on their different affinities for a stationary and a mobile phase. The mixture is passed through a stationary phase, and the different substances move at different rates, allowing them to be separated.
Properties of Matter
Matter can be characterized by its physical and chemical properties.
Physical propertiesare those that can be observed or measured without changing the substance’s chemical composition. Examples include density, melting point, and boiling point.
Chemical propertiesdescribe the substance’s ability to undergo chemical reactions. Examples include flammability, reactivity, and toxicity.
Properties of matter can be used to identify and classify substances, as well as to predict their behavior in different situations.
FAQ Overview
What is the difference between a physical change and a chemical change?
A physical change alters the form or appearance of a substance without changing its chemical composition, while a chemical change involves the rearrangement of atoms to form new substances with different properties.
How can I identify an element?
Elements are pure substances that cannot be broken down into simpler substances by chemical means. They are identified by their unique atomic number, which represents the number of protons in their nuclei.
What is the principle behind distillation?
Distillation is a separation technique that utilizes the different boiling points of liquids. By heating a mixture, the lower-boiling component vaporizes first and can be condensed into a separate container.